26 Oct 2024 14:20:51 pm
Tags : Presbyopia
Topic: Health Issues
Why in the news?
Source: The Hindu
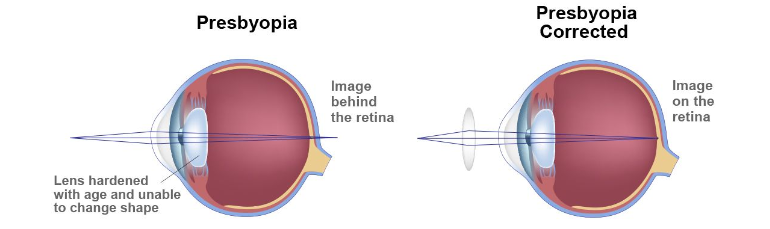
About Presbyopia:
Central Drugs Standard Control Organisation (CDSCO):
|
0 Comments

© 2026 Catalyst IAS All Rights Reserved.